Multiple Choice
Consider the figure that shows ΔG° for a chemical process plotted against absolute temperature. From this plot, it is reasonable to conclude that 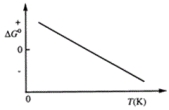
A) ΔH° > 0, ΔS° > 0
B) ΔH° > 0, ΔS° < 0
C) ΔH° < 0, ΔS° > 0
D) ΔH° < 0, ΔS° < 0
E) None of these choices are correct.
Correct Answer:
Verified
Related Questions
Q4: As a chemical reaction proceeds toward equilibrium,
Q9: For any reaction, if ΔG° > 0,
Q10: The higher the pressure of a gas
Q14: For a reaction at equilibrium, ΔSuniv =
Q76: A reaction has ΔG = 10.0 kJ
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents