Safrole is used as a topical antiseptic. Calculate the vapor pressure of a solution prepared by dissolving 0.75 mol of safrole in 950 g of ethanol ( 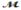 = 46.07 g/mol) . P°ethanol = 50.0 torr at 25°C.
= 46.07 g/mol) . P°ethanol = 50.0 torr at 25°C.
A) 1.8 torr
B) 11 torr
C) 15 torr
D) 40 torr
E) 48 torr
Correct Answer:
Verified
Q17: All solutions are mixtures
Q67: Which one of the following pairs of
Q68: Benzaldehyde ( Q74: Calculate the vapor pressure of a solution Q75: Determine the freezing point of a solution Q76: Which of the following aqueous liquids will Q77: A 0.100 m MgSO4 solution has a Q84: Lysine is an amino acid that is Q88: An emulsion is a dispersion consisting of Q92: The Tyndall effect![]()
A)is observed in concentrated solutions.
B)is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents