Barbiturates are synthetic drugs used as sedatives and hypnotics. Barbital ( 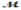 = 184.2 g/mol) is one of the simplest of these drugs. What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid? Kb = 3.07°C/m; boiling point of pure acetic acid = 117.9°C
= 184.2 g/mol) is one of the simplest of these drugs. What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid? Kb = 3.07°C/m; boiling point of pure acetic acid = 117.9°C
A) 117.0°C
B) 117.7°C
C) 118.1°C
D) 118.8°C
E) >120°C
Correct Answer:
Verified
Q59: Children under the age of 6 with
Q61: How many moles of bromide ions are
Q62: Cinnamaldehyde ( Q64: Calculate the freezing point of a solution Q65: Hexachlorophene is used as a disinfectant in Q68: From the following list of aqueous solutions Q78: Which of the following aqueous solutions should Q80: Two aqueous are prepared: 1.00 m Na2CO3 Q95: Human blood has a molar concentration of Q96: A 0.100 m K2SO4 solution has a![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents