Multiple Choice
Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 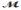 = 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
= 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
A) -1.7°C
B) -0.9°C
C) 0.0°C
D) 0.9°C
E) 1.7°C
Correct Answer:
Verified
Related Questions
Q59: Children under the age of 6 with
Q61: Barbiturates are synthetic drugs used as sedatives
Q62: Cinnamaldehyde ( Q65: Hexachlorophene is used as a disinfectant in Q67: Which one of the following pairs of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents