According to valence bond theory, the triple bond in ethyne (acetylene, C2H2) consists of
A) three σ bonds and no 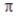 bonds.
bonds.
B) two σ bonds and one 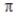 bond.
bond.
C) one σ bond and two 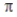 bonds.
bonds.
D) no σ bonds and three 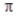 bonds.
bonds.
E) None of these choices are correct.
Correct Answer:
Verified
Q16: Which one of the following statements about
Q18: The angles between sp2 hybrid orbitals are
Q24: Overlap of two sp2 hybrid orbitals produces
Q26: Which of the following statements relating to
Q27: Valence bond theory predicts that bromine will
Q28: Valence bond theory predicts that iodine will
Q29: When PCl5 solidifies it forms PCl4+ cations
Q35: Determine the shape (geometry) of PCl3 and
Q39: A molecule with the formula AX4E uses
Q46: According to molecular orbital theory, what is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents