Which of the following statements relating to molecular orbital (MO) theory is incorrect?
A) Combination of two atomic orbitals produces one bonding and one antibonding MO.
B) A bonding MO is lower in energy than the two atomic orbitals from which it is formed.
C) Combination of two 2p orbitals may result in either σ or 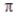 MOs.
MOs.
D) A species with a bond order of zero will not be stable.
E) In a stable molecule having an even number of electrons, all electrons must be paired.
Correct Answer:
Verified
Q3: According to valence bond theory, overlap of
Q11: Hybrid orbitals of the sp3d type occur
Q18: The angles between sp2 hybrid orbitals are
Q19: In the valence bond treatment, overlap of
Q21: According to valence bond theory, the triple
Q24: Overlap of two sp2 hybrid orbitals produces
Q35: Determine the shape (geometry) of PCl3 and
Q42: Which one, if any, of the following
Q45: The nitrosonium ion, NO+, forms a number
Q46: According to molecular orbital theory, what is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents