The electrochemical reaction that powers a lead-acid storage battery is as follows: Pb(s) + PbO2(s) + 4 H+(aq) + 2 SO42-(aq) → 2 PbSO4(s) + 2 H2O( 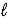 )
)
A single cell of this battery consists of a Pb electrode and a PbO2 electrode,each submerged in sulfuric acid.Which of the following reactions occurs at the cathode during discharge?
A) Pb(s) is reduced to PbSO4(s) .
B) PbO2(s) is reduced to PbSO4(s) .
C) PbO2(s) is oxidized to PbSO4(s) .
D) Pb(s) is oxidized to PbSO4(s) .
E) H+ is oxidized to H2O( 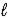 ) .
) .
Correct Answer:
Verified
Q16: When the given oxidation-reduction reaction in an
Q17: Write the balanced reduction half-reaction for the
Q18: Write a balanced chemical equation for the
Q19: In the context of the diagram given
Q20: Which of the following is the cell
Q22: Given the following two half-reactions,write the overall
Q23: Write a balanced net ionic equation for
Q24: An SHE electrode has been assigned a
Q25: Calculate the standard cell potential (
Q26: In the given electrochemical cell,which of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents