An SHE electrode has been assigned a standard reduction potential,E°,of 0.00 volts.Which of the following reactions will occur at this electrode?
A) 2 H2O( 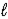 ) + 2 e- → H2(g) + 2 OH-(aq)
) + 2 e- → H2(g) + 2 OH-(aq)
B) O2(g) + 4 e- → 2 O2-(aq)
C) Hg2Cl2(s) + 2 e- → 2 Hg( 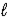 ) + 2 Cl-(aq)
) + 2 Cl-(aq)
D) Li+(aq) + e- → Li(s)
E) 2 H+(aq) + 2 e- → H2(g)
Correct Answer:
Verified
Q19: In the context of the diagram given
Q20: Which of the following is the cell
Q21: The electrochemical reaction that powers a lead-acid
Q22: Given the following two half-reactions,write the overall
Q23: Write a balanced net ionic equation for
Q25: Calculate the standard cell potential (
Q26: In the given electrochemical cell,which of the
Q27: Calculate E°cell for the cell for the
Q28: Use the following standard reduction potentials to
Q29: Which of the following species are likely
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents