Which of the following is the balanced overall reaction and standard cell potential of an electrochemical cell constructed from half-cells with the given half reactions? Pt2+(aq) + 2 e− → Pt(s) ; E° = 1.180 V
Pb2+(aq) + 2 e− → Pb(s) ; E° = -0.130 V
A) Pt2+(aq) + Pb(s) → Pt(s) + Pb2+(aq) ; 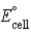 = 1.310 V
= 1.310 V
B) Pt(s) + Pb2+(aq) → Pt2+(aq) + Pb(s) ; 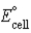 = −1.310 V
= −1.310 V
C) Pt2+(aq) + Pb2+(aq) → Pt(s) + Pb(s) ; 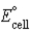 = 1.050 V
= 1.050 V
D) Pt2+(aq) + Pb(s) → Pt(s) + Pb2+(aq) ; 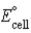 =0.655 V
=0.655 V
E) Pt(s) + Pb2+(aq) → Pt2+(aq) + Pb(s) ; 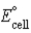 = −0.655 V
= −0.655 V
Correct Answer:
Verified
Q28: Use the following standard reduction potentials to
Q29: Which of the following species are likely
Q30: The unit for electromotive force,emf,is the volt.1
Q31: According to the cell notation below,which of
Q32: Use the following standard reduction potentials to
Q34: Consider the following half-reactions. Ag+(aq)+ e- →
Q35: Use the following standard reduction potentials to
Q36: Consider the following half-reactions. Cl2(g)+ 2 e-
Q37: Which of the following is the cell
Q38: Write a balanced net ionic equation for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents