Calculate ΔrG° at 25.0 °C for the reaction below. 2 Na(s) + 2 H2O( 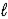 ) → 2 NaOH(aq) + H2(g)
) → 2 NaOH(aq) + H2(g)
Given: ΔrH° = −366.6 kJ/mol-rxn and ΔrS° = −154.2 J/K⋅mol-rxn.
A) -371.2 kJ/mol-rxn
B) -320.6 kJ/mol-rxn
C) -215.4 kJ/mol-rxn
D) +371.2 kJ/mol-rxn
E) +4634.9 kJ/mol-rxn
Correct Answer:
Verified
Q25: If ΔrG° > 0 for a reaction
Q26: Calculate ΔG° at 298 K for the
Q27: A change of state occurring in a
Q28: If a chemical reaction is exothermic but
Q29: At what temperatures will a reaction be
Q31: ΔG° = 0 for a reaction indicates
Q32: A flask containing helium gas is released
Q33: If a cube of ice at 0
Q34: For a reaction,ΔrH° = -208.8 kJ and
Q35: Hydrogen gas is prepared by electrolysis of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents