Given the two equilibria below, Ag(NH3) 2+(aq) 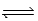 Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8
Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8
AgCN(s) 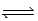 Ag+(aq) + CN−(aq) ; Ksp =
Ag+(aq) + CN−(aq) ; Ksp = 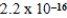 what is K for the following equilibrium?
what is K for the following equilibrium?
AgCN(s) + 2NH3(aq)  Ag(NH3) 2+(aq) + CN-(aq)
Ag(NH3) 2+(aq) + CN-(aq)
A) 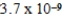
B) 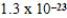
C) 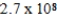
D) 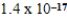
E) 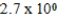
Correct Answer:
Verified
Q64: Two important biological buffer systems control pH
Q65: What is the molar solubility of Mn(OH)2(s)in
Q66: If the ratio of acid to base
Q67: A 5.0 × 10-4 M solution of
Q68: Consider the reaction Cu2+(aq)+ 4 NH3(aq)
Q70: What is the concentration of Cd2+(aq)in a
Q71: Suppose 50.00 mL of 2.0 × 10-5
Q72: The concentration of Pb2+ in an aqueous
Q73: An aqueous solution contains 0.010 M bromide
Q74: Given the following reactions, AgBr(s) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents