Given the following reactions, AgBr(s) 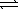 Ag+(aq) + Br-(aq)
Ag+(aq) + Br-(aq)
Ksp = 5.4 × 10-13
Ag+(aq) + 2 CN-(aq) 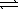 Ag(CN) 2-(aq)
Ag(CN) 2-(aq)
Kf = 1.2 × 1021
Determine the equilibrium constant for the reaction below.
AgBr(s) + 2 CN-(aq) 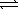 Ag(CN) 2-(aq) + Br-(aq)
Ag(CN) 2-(aq) + Br-(aq)
A) 4.5 × 10-34
B) 1.5 × 10-9
C) 6.5 × 108
D) 1.2 × 1021
E) 2.2 × 1033
Correct Answer:
Verified
Q69: Given the two equilibria below, Ag(NH3)2+(aq)
Q70: What is the concentration of Cd2+(aq)in a
Q71: Suppose 50.00 mL of 2.0 × 10-5
Q72: The concentration of Pb2+ in an aqueous
Q73: An aqueous solution contains 0.010 M bromide
Q75: Calculate the molar concentration of uncomplexed Zn2+(aq)in
Q76: What is the value of the dissociation
Q77: If 500 mL of 1.4 × 10-6
Q78: What is the maximum hydroxide-ion concentration that
Q79: For a monoprotic acid titration,the _ in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents