Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.17 M Zn(NH3) 42+ and 0.3775 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 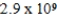 .
.
A) 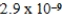 M
M
B) 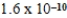 M
M
C) 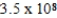 M
M
D) 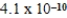 M
M
E) 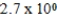 M
M
Correct Answer:
Verified
Q70: What is the concentration of Cd2+(aq)in a
Q71: Suppose 50.00 mL of 2.0 × 10-5
Q72: The concentration of Pb2+ in an aqueous
Q73: An aqueous solution contains 0.010 M bromide
Q74: Given the following reactions, AgBr(s) 
Q76: What is the value of the dissociation
Q77: If 500 mL of 1.4 × 10-6
Q78: What is the maximum hydroxide-ion concentration that
Q79: For a monoprotic acid titration,the _ in
Q80: What is the minimum concentration of Cd2+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents