Given the following equilibrium constants, Zn4IO3) 2 Ksp = 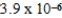 Zn(NH3) 42+ Kf =
Zn(NH3) 42+ Kf = 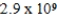 determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq) ⇌ Zn(NH3) 42+(aq) + 2IO3-(aq)
A) 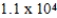
B) 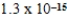
C) 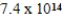
D) 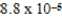
E) 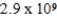
Correct Answer:
Verified
Q58: A 25.0 mL sample of 0.10 M
Q59: In which of the following solutions would
Q59: Titration of 0.1615 g of an unknown
Q60: An impure sample of sodium carbonate,Na2CO3,is titrated
Q62: What is the minimum mass of Na2CO3
Q64: Two important biological buffer systems control pH
Q65: What is the molar solubility of Mn(OH)2(s)in
Q66: If the ratio of acid to base
Q67: A 5.0 × 10-4 M solution of
Q68: Consider the reaction Cu2+(aq)+ 4 NH3(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents