Given that Ka for the weak acid HA is 3.49 × 10-8,calculate K for the reaction of HA with OH-. HA(aq) + OH−(aq) D A−(aq) + H2O( 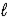 )
)
A) 3.49
B) 3.49 × 106
C) 3.49 × 10-22
D) 2.87 × 1021
E) 2.87 × 10-7
Correct Answer:
Verified
Q37: The pH of a solution at 25
Q38: What is the pOH of 0.074 M
Q39: Hydrofluoric acid has a pKa value of
Q40: What is the H3O+ concentration in 0.0055
Q41: H3PO3 is a diprotic weak acid.What is
Q43: The equilibrium constant,Ka,for a monoprotic acid (benzoic
Q44: Consider the Ka values for the following
Q45: Which acid-base reaction results in acidic solution?
A)
Q46: Given the following acid dissociation constants: Ka
Q47: Given the following equilibrium constants, Ka (HSO4-)=
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents