Given the following acid dissociation constants: Ka (H3PO4) = 7.5 × 10-3
Ka (NH4+) = 5.6 × 10-10
Determine the equilibrium constant for the reaction below at 25 °C.
H3PO4(aq) + NH3(aq) 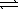 NH4+(aq) + H2PO4−(aq)
NH4+(aq) + H2PO4−(aq)
A) 4.2 × 10-12
B) 7.5 × 10-8
C) 4.2 × 102
D) 1.3 × 107
E) 2.4 × 1011
Correct Answer:
Verified
Q41: H3PO3 is a diprotic weak acid.What is
Q42: Given that Ka for the weak acid
Q43: The equilibrium constant,Ka,for a monoprotic acid (benzoic
Q44: Consider the Ka values for the following
Q45: Which acid-base reaction results in acidic solution?
A)
Q47: Given the following equilibrium constants, Ka (HSO4-)=
Q48: Which of the following ionic compounds forms
Q49: What is the equilibrium constant for the
Q50: Which of the following ionic compounds does
Q51: What is the equilibrium pH of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents