At 25 °C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below? AgBr(s) 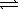 Ag+(aq) + Br-(aq)
Ag+(aq) + Br-(aq)
A) 5.40 × 10-13
B) 5.40 × 10-11
C) 1.90 × 10-8
D) 7.35 × 10-7
E) 1.90 × 10-6
Correct Answer:
Verified
Q31: When 0.20 mole HF is dissolved in
Q32: For the reaction given below,2.00 moles of
Q33: What is the reaction quotient,Q,for the equilibrium
Q34: A sample of solid NH4NO3 was placed
Q35: Excess Ag2SO4(s)is placed in water at 25
Q37: At a high temperature,equal concentrations of 0.160
Q38: A 2.5 L flask is filled with
Q39: Which of the following statements about the
Q40: A 3.00-liter flask initially contains 3.00 mol
Q41: The equilibrium constant (Kc)for the decomposition of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents