Consider the following equilibria. PbBr2(s) 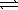 Pb2+(aq) + 2 Br-(aq)
Pb2+(aq) + 2 Br-(aq)
K1 = 6.6 × 10-6
Pb(OH) 2(s) 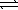 Pb2+(aq) + 2 OH-(aq)
Pb2+(aq) + 2 OH-(aq)
K2 = 1.4 × 10-15
Determine the equilibrium constant,Kc,for the reaction below.
PbBr2(s) + 2 OH-(aq) 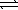 Pb(OH) 2(s) + 2 Br-(aq)
Pb(OH) 2(s) + 2 Br-(aq)
A) 9.2 × 10-21
B) 2.1 × 10-10
C) 6.6 × 10-6
D) 4.7 × 109
E) 1.1 × 1020
Correct Answer:
Verified
Q60: Consider the reaction H2 + I2
Q61: In 1913,the Haber-Bosch process was patented.The product
Q62: The thermochemical equation for the formation of
Q63: In which of the following reactions does
Q64: Consider the following equilibrium. PCl3(g)+ Cl2(g)
Q66: Given the equilibrium constants for the following
Q67: If a stress is applied to an
Q68: The symbol Q is called the _.
Q69: When the pressure of an equilibrium mixture
Q70: Which of the following statements is true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents