In which of the following reactions does a decrease in the volume of the reaction vessel at constant temperature favor the formation of the products?
A) 2 H2(g) + O2(g) 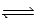 2 H2O(g)
2 H2O(g)
B) NO2(g) + CO(g) 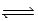 NO(g) + CO2(g)
NO(g) + CO2(g)
C) H2(g) + I2(g) 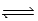 2 HI(g)
2 HI(g)
D) 2 O3(g) 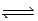 3 O2(g)
3 O2(g)
E) MgCO3(s) 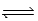 MgO(s) + CO2(g)
MgO(s) + CO2(g)
Correct Answer:
Verified
Q58: For the equilibrium PCl5(g) Q59: If Kc = 0.124 for A2 + Q60: Consider the reaction H2 + I2 Q61: In 1913,the Haber-Bosch process was patented.The product Q62: The thermochemical equation for the formation of Q64: Consider the following equilibrium. PCl3(g)+ Cl2(g) Q65: Consider the following equilibria. PbBr2(s) Q66: Given the equilibrium constants for the following Q67: If a stress is applied to an Q68: The symbol Q is called the _.![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents