For the equilibrium PCl5(g) 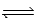 PCl3(g) + Cl2(g) ,Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g) is 0.19 M,what is the equilibrium concentration of PCl3?
PCl3(g) + Cl2(g) ,Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g) is 0.19 M,what is the equilibrium concentration of PCl3?
A) 0.095 M
B) 0.4 M
C) 0.19 M
D) 0.87 M
E) 0.009 M
Correct Answer:
Verified
Q53: At 700 K,Kp for the following equilibrium
Q54: At 25 °C,the decomposition of dinitrogen tetraoxide
Q55: A mixture of nitrogen and hydrogen was
Q56: The equilibrium constant at 25 °C for
Q57: At 800 K,the equilibrium constant,Kp,for the following
Q59: If Kc = 0.124 for A2 +
Q60: Consider the reaction H2 + I2
Q61: In 1913,the Haber-Bosch process was patented.The product
Q62: The thermochemical equation for the formation of
Q63: In which of the following reactions does
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents