At 700 K,Kp for the following equilibrium is 5.6 × 10-3. 2HgO(s) 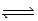 2Hg(l) + O2(g)
2Hg(l) + O2(g)
Suppose 43.1 g of mercury(II) oxide is placed in a sealed 4.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L · atm/(K · mol) )
A) 0.074 atm
B) 0.0056 atm
C) 2.8 atm
D) 14 atm
E) 1.4 atm
Correct Answer:
Verified
Q48: In an experiment,0.46 mol H2 and 0.46
Q49: For the reaction N2O4(g) Q50: A 3.50-mol sample of HI is placed Q51: Consider the following equilibrium: CO2(g)+ H2(g) Q52: For the equilibrium PCl5(g) Q54: At 25 °C,the decomposition of dinitrogen tetraoxide Q55: A mixture of nitrogen and hydrogen was Q56: The equilibrium constant at 25 °C for Q57: At 800 K,the equilibrium constant,Kp,for the following Q58: For the equilibrium PCl5(g) Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

