At 800 K,the equilibrium constant,Kp,for the following reaction is 3.2 × 10-7. 2 H2S(g) 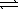 2 H2(g) + S2(g)
2 H2(g) + S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
A) 8.5 × 10-5 atm
B) 6.2 × 10-3 atm
C) 9.0 × 10-3 atm
D) 1.1 × 10-2 atm
E) 1.4 × 10-2 atm
Correct Answer:
Verified
Q52: For the equilibrium PCl5(g) Q53: At 700 K,Kp for the following equilibrium Q54: At 25 °C,the decomposition of dinitrogen tetraoxide Q55: A mixture of nitrogen and hydrogen was Q56: The equilibrium constant at 25 °C for![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents