Assume that the following chemical reaction is at equilibrium. 2 ICl(g) 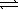 I2(g) + Cl2(g)
I2(g) + Cl2(g)
ΔH° = +26.9 kJ
At 25 °C,Kp = 2.0 × 105.If the temperature is increase to 45 °C,which statement applies?
A) Kp will decrease and the reaction will proceed in the backward direction.
B) Kp will decrease and the reaction will proceed in the forward direction.
C) Kp will remain unchanged and the reaction will proceed in the forward direction.
D) Kp will increase and the reaction will proceed in the backward direction.
E) Kp will increase and the reaction will proceed in the forward direction.
Correct Answer:
Verified
Q67: If a stress is applied to an
Q68: The symbol Q is called the _.
Q69: When the pressure of an equilibrium mixture
Q70: Which of the following statements is true
Q71: The equilibrium constant,K,is always the same within
Q72: When 1.0 mole of acetic acid is
Q73: Which of the following equilibria would not
Q74: Given the following equilibria, Ni2+(aq)+ 2 OH-(aq)
Q75: The standard enthalpy of formation of ammonia
Q76: Given the equilibrium constants for the equilibria,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents