What volume of 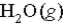 measured at STP is produced by the combustion of 6.50 g of natural gas
measured at STP is produced by the combustion of 6.50 g of natural gas 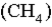 in excess oxygen? (R = 0.08206 L⋅atm/mol⋅K)
in excess oxygen? (R = 0.08206 L⋅atm/mol⋅K) 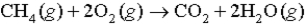
A) 4.54 L
B) 20 L
C) 9.08 L
D) 18.2 L
E) 3.25 L
Correct Answer:
Verified
Q50: A 3.98 gram sample of a certain
Q51: Sodium azide decomposes rapidly to produce nitrogen
Q52: A 22.4 L high pressure reaction vessel
Q53: One way to isolate metals from their
Q54: The density of ethane,C2H6 (30.1 g/mol),at 27°C
Q56: When 0.5000 grams of an unknown hydrocarbon,CxHy,is
Q57: An unknown gaseous hydrocarbon contains 85.63% C.Its
Q58: Ammonia gas is synthesized according to the
Q59: Aqueous hydrochloric acid reacts with magnesium to
Q60: What is the total volume of gases
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents