One way to isolate metals from their ores is by a chemical reaction of the metal oxide with carbon as shown below (M = metal) : 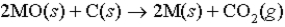 If 31.75 g of a metal oxide reacts with excess carbon to form 4.07 L of CO2 at 100°C and 1.50 atm,what is the identity of the metal?
If 31.75 g of a metal oxide reacts with excess carbon to form 4.07 L of CO2 at 100°C and 1.50 atm,what is the identity of the metal?
A) Hg
B) Mg
C) Cu
D) Pb
E) Cd
Correct Answer:
Verified
Q48: What volume of O2,measured at 24.0°C and
Q49: Which of the following samples contains the
Q50: A 3.98 gram sample of a certain
Q51: Sodium azide decomposes rapidly to produce nitrogen
Q52: A 22.4 L high pressure reaction vessel
Q54: The density of ethane,C2H6 (30.1 g/mol),at 27°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents