What volume of O2,measured at 24.0°C and 0.882 atm atm,will be produced by the decomposition of 3.00 g KClO3? Assume 100% yield.(R = 0.08206 L⋅atm/mol⋅K) 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
A) 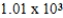 mL
mL
B) 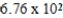 mL
mL
C) 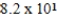 mL
mL
D) 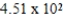 mL
mL
E) none of these
Correct Answer:
Verified
Q43: The following equation represents the partial combustion
Q44: A mixture of KCl and KClO3 weighing
Q45: Into a 2.82-liter container at 25°C are
Q46: Under conditions of constant volume and moles
Q47: The density of a gas is 1.25
Q49: Which of the following samples contains the
Q50: A 3.98 gram sample of a certain
Q51: Sodium azide decomposes rapidly to produce nitrogen
Q52: A 22.4 L high pressure reaction vessel
Q53: One way to isolate metals from their
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents