A mixture of KCl and KClO3 weighing 1.65 grams was heated; the dry O2 generated occupied 143 mL at STP.What percent by mass of the original mixture was KClO3,which decomposes as follows: 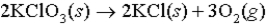
A) 31.6%
B) 47.4%
C) 71.1%
D) 8.67%
E) 19.2%
Correct Answer:
Verified
Q39: What volume of gaseous silane,SiH4,has the same
Q40: What volume does 49.0 g of N2
Q41: The density of H2 gas in a
Q42: An excess of sodium hydroxide is treated
Q43: The following equation represents the partial combustion
Q45: Into a 2.82-liter container at 25°C are
Q46: Under conditions of constant volume and moles
Q47: The density of a gas is 1.25
Q48: What volume of O2,measured at 24.0°C and
Q49: Which of the following samples contains the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents