Determine the standard enthalpy of formation of Fe2O3(s) given the thermochemical equations below. Fe(s) + 3 H2O( 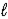 ) → Fe(OH) 3(s) + 3/2 H2(g)
) → Fe(OH) 3(s) + 3/2 H2(g)
ΔrH° = +160.9 kJ/mol-rxn
H2(g) + 1/2 O2(g) → H2O( 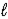 )
)
ΔrH° = -285.8 kJ/mol-rxn
Fe2O3(s) + 3 H2O( 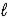 ) → 2 Fe(OH) 3(s)
) → 2 Fe(OH) 3(s)
ΔrH° = +288.6 kJ/mol-rxn
A) -252.6 kJ/mol-rxn
B) +163.7 kJ/mol-rxn
C) -824.2 kJ/mol-rxn
D) +33.2 kJ/mol-rxn
E) + 890.6 kJ/mol-rxn
Correct Answer:
Verified
Q40: Calculate ΔU of a gas for a
Q41: When 50.0 mL of 1.60 M of
Q42: What is the overall chemical equation that
Q43: Determine ΔrH° for the following reaction,2 NH3(g)+
Q44: A chemical reaction in a bomb calorimeter
Q46: A bomb calorimeter has a heat capacity
Q47: Which of the following reactions corresponds to
Q48: Determine the heat of evaporation of carbon
Q49: Determine the standard enthalpy of formation of
Q50: In thermodynamics,a(n)_ is defined as the object,or
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents