Write a balanced equation for the reaction that occurs when hydrogen sulfate ion behaves as a Brønsted-Lowry acid in water.
A) HSO4-(aq) + H2O( 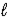 )
) 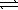 H2SO4(aq) + H3O+(aq)
H2SO4(aq) + H3O+(aq)
B) SO42-(aq) + H2O( 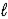 )
) 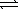 HSO4-(aq) + OH-(aq)
HSO4-(aq) + OH-(aq)
C) HSO4-(aq) + H2O( 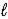 )
) 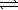 SO42-(aq) + H3O+(aq)
SO42-(aq) + H3O+(aq)
D) HSO4-(aq) + H2O( 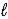 )
) 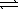 H2SO4(aq) + OH-(aq)
H2SO4(aq) + OH-(aq)
E) H2SO4(aq) + H2O( 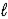 )
) 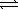 HSO4-(aq) + H3O+(aq)
HSO4-(aq) + H3O+(aq)
Correct Answer:
Verified
Q19: All of the following compounds are soluble
Q20: Which of the following compounds is soluble
Q21: Which of the following would not be
Q22: Which of the following compounds acts as
Q23: If an aqueous solution of _ is
Q25: Which of the following compounds is a
Q26: Write a balanced chemical equation for the
Q27: Which of the following compounds cannot be
Q28: Which of the following is a weak
Q29: Which of the following products will form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents