A small birthday candle is weighed.It is then lighted and placed beneath a metal can containing 100 mL of water.Careful records are kept as the temperature of the water rises.Data from this experiment are shown on the graph in Figure 2.9.What amount of heat energy is released in the burning of candle wax? (Note that 1 liter of pure water has a mass of 1 kg. ) 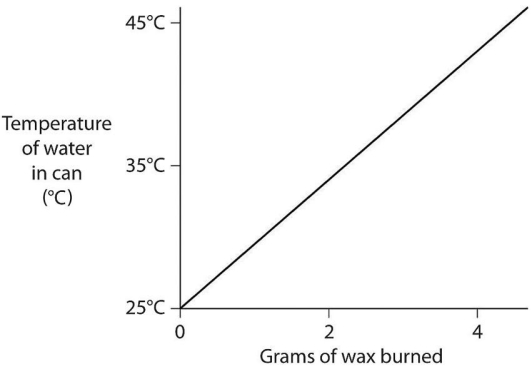 Figure 2.9
Figure 2.9
A) 0.5 kilocalorie per gram of wax burned
B) 5 kilocalories per gram of wax burned
C) 10 kilocalories per gram of wax burned
D) 20 kilocalories per gram of wax burned
E) 50 kilocalories per gram of wax burned
Correct Answer:
Verified
Q41: We can be sure that a mole
Q65: You have two beakers. One contains a
Q125: Scientists have synthesized a new artificial compound
Q126: Carbon dioxide (CO2)is readily soluble in water,according
Q128: Many mammals control their body temperature by
Q129: Measurements show that the pH of a
Q130: Identical heat lamps are arranged to shine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents