Identify a balanced reaction for the smelting of SnO2 with carbon.
A) SnO2(s) + CO2(g) 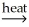 SnCO3(s)
SnCO3(s)
B) SnO2(s) + CaCO3(s) 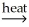 SnC(s) + CaO(s) + O2(g)
SnC(s) + CaO(s) + O2(g)
C) SnO2(s) + 2C(s) 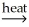 Sn(l) + 2CO(g)
Sn(l) + 2CO(g)
D) 2SnO2(s) + 2CaC2(s) 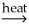 2SnC2(s) + 2CaO(s) + O2(g)
2SnC2(s) + 2CaO(s) + O2(g)
E) SnO2(s) + 2CO(g) 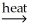 Sn(l) + 2CO2(g)
Sn(l) + 2CO2(g)
Correct Answer:
Verified
Q16: Which of the following is TRUE of
Q17: Which of the following is an example
Q18: Pyrometallurgy is the _.
A) refining of metal
Q19: Which of the following is an example
Q20: Which of the following describes flux?
A) a
Q23: Which of the following is TRUE concerning
Q24: Why can't chromium and nickel form a
Q25: What are the components of brass?
A) copper
Q26: Identify a balanced equation for the calcination
Q29: An interstitial alloy contains carbon in one-fourth
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents