Identify a balanced equation for the calcination of NiCO3.
A) NiCO3(s) + CaO(s) 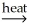 NiO(s) + CaCO3(s)
NiO(s) + CaCO3(s)
B) NiCO3(s) 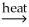 NiO(s) + CO2(g)
NiO(s) + CO2(g)
C) NiCO3(s) + C(s) 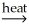 NiO(s) + 2CO(g)
NiO(s) + 2CO(g)
D) 2NiCO3(s) 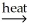 2Ni(l) + 2CO2(g) + O2(g)
2Ni(l) + 2CO2(g) + O2(g)
E) NiCO3(s) + CO(g) 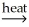 NiO(s) + 2CO2(g)
NiO(s) + 2CO2(g)
Correct Answer:
Verified
Q21: Identify a balanced reaction for the smelting
Q23: Which of the following is TRUE concerning
Q24: Why can't chromium and nickel form a
Q25: What are the components of brass?
A) copper
Q26: An interstitial alloy contains hydrogen in half
Q28: Which of the following is TRUE concerning
Q29: An interstitial alloy contains carbon in one-fourth
Q29: Which of the following is an example
Q30: Match the following.
-Hg
A)malachite
B)rutile
C)carnotite
D)sphalerite
E)cinnabar
F)rhodochrosite
G)galena
Q31: What are the components of bronze?
A) copper
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents