A voltaic cell is constructed with two 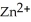 -Zn electrodes, where the half-reaction is
-Zn electrodes, where the half-reaction is 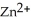 +
+ 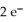 → Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 1.17 ×
→ Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 1.17 × 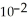 mol L-1 and 8.70 ×
mol L-1 and 8.70 × 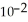 mol L-1, respectively. The cell emf is ________ V.
mol L-1, respectively. The cell emf is ________ V.
A) 0.170
B) -0.678
C) -0.0850
D) 515
E) 0.0850
Correct Answer:
Verified
Q99: What is the shorthand notation that represents
Q100: Identify the metal if during a 1.000
Q101: The standard emf for the cell using
Q103: How many seconds are required to produce
Q106: A voltaic cell is constructed with two
Q108: The standard emf for the cell using
Q121: How many grams of chromium metal are
Q124: For a galvanic cell that uses the
Q125: How long must a constant current of
Q127: How many grams of nickel metal are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents