How many seconds are required to produce 7.00 g of aluminum metal from the electrolysis of molten 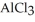 with an electrical current of 18.0 A?
with an electrical current of 18.0 A?
A) 1.39 × 103 s
B) 1.35 × 106 s
C) 10.5 s
D) 23.1 s
E) 4.17 × 103 s
Correct Answer:
Verified
Q99: What is the shorthand notation that represents
Q100: Identify the metal if during a 1.000
Q101: The standard emf for the cell using
Q104: A voltaic cell is constructed with two
Q106: A voltaic cell is constructed with two
Q108: The standard emf for the cell using
Q121: How many grams of chromium metal are
Q124: For a galvanic cell that uses the
Q125: How long must a constant current of
Q151: Based on the following information,
Cl2(g)+ 2 e-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents