In the Haber process, ammonia is synthesized from nitrogen and hydrogen: 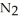 (g) +
(g) + 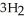 (g) →
(g) → 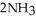 (g) ΔrG° at 298 K for this reaction is -33.3 kJ mol-1. The value of ΔrG at 298 K for a reaction mixture that consists of 1.7 atm
(g) ΔrG° at 298 K for this reaction is -33.3 kJ mol-1. The value of ΔrG at 298 K for a reaction mixture that consists of 1.7 atm 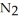 , 1.1 atm
, 1.1 atm 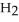 , and 0.55 atm
, and 0.55 atm 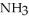 is ________ kJ mol-1.
is ________ kJ mol-1.
A) -5.02 × 103
B) -4.5
C) -72.2
D) -35.3
E) -38.3
Correct Answer:
Verified
Q107: Match the following.
-Q = K
A)standard state
B)equilibrium
C)ΔrG >
Q107: Match the following.
-Q = K
A)standard state
B)equilibrium
C)ΔrG >
Q108: The value of ΔfG° at 400.0 °C
Q109: Determine the Q of a reaction at
Q110: Match the following.
-Q = 1
A)standard state
B)equilibrium
C)ΔrG >
Q111: Calculate the equilibrium constant for the following
Q113: Determine the equilibrium constant for the following
Q115: For a given reaction, ΔrH = -19.5
Q116: The value of ΔfG° at 161.0 °C
Q117: Calculate the equilibrium constant for the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents