Calculate the percent ionization of nitrous acid in a solution that is 0.210 mol L-1 in nitrous acid 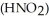 and 0.290 mol L-1 in potassium nitrite (
and 0.290 mol L-1 in potassium nitrite ( 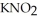 ) . The acid dissociation constant of nitrous acid is
) . The acid dissociation constant of nitrous acid is 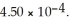
A) 58.0
B) 0.154
C) 16.6
D) 2.74 × 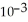
E) 1.55
Correct Answer:
Verified
Q117: What is the pH of a buffer
Q134: A 25.0 mL sample of 0.150 mol
Q135: What is the pH of a buffer
Q137: How many millilitres of 0.120 mol L-1
Q138: What is the pH of a solution
Q140: What is the pH of a solution
Q141: Solid potassium chromate (K2CrO4) is slowly added
Q142: What is the molar solubility of AgCl
Q143: What is the pH at the equivalence
Q144: Match the following.
-pH = 7
A)equivalence point of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents