Solid potassium chromate (K2CrO4) is slowly added to a solution containing 0. 50 mol L-1 AgNO3 and 0. 50 mol L-1 Ba(NO3) 2. What is the Ag+ concentration when BaCrO4 just starts to precipitate? The Ksp for Ag2CrO4 and BaCrO4 are 1.1 × 10-12 and 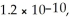 respectively.
respectively.
A) 6.5 × 10-5 mol L-1
B) 1.3 × 10-4 mol L-1
C) 3.2 × 10-4 mol L-1
D) 6.8 × 10-2 mol L-1
Correct Answer:
Verified
Q117: What is the pH of a buffer
Q137: How many millilitres of 0.120 mol L-1
Q138: What is the pH of a solution
Q139: Calculate the percent ionization of nitrous acid
Q140: What is the pH of a solution
Q142: What is the molar solubility of AgCl
Q143: What is the pH at the equivalence
Q144: Match the following.
-pH = 7
A)equivalence point of
Q145: A solution of NaF is added dropwise
Q146: Calculate the solubility (in g L-1) of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents