A solution of methanol and water has a vapour pressure of 213 Torr. What would you predict as the mole fractions of each component, assuming ideal behaviour? The vapour pressures of methanol and water are 256 Torr and 55.3 Torr, respectively.
A) 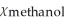 = 0.214,
= 0.214,  = 0.786
= 0.786
B) 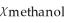 = 0.786,
= 0.786,  = 0.214
= 0.214
C) 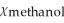 = 0.885,
= 0.885,  = 0.115
= 0.115
D) 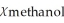 = 0.900,
= 0.900,  = 0.100
= 0.100
E) 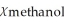 = 0.115,
= 0.115,  = 0.885
= 0.885
Correct Answer:
Verified
Q54: Fluids used for an intravenous transfusion must
Q56: Solutions having osmotic pressures less than those
Q101: A solution is 2.25% by weight NaHCO3.How
Q112: What is the mole fraction of oxygen
Q114: What is the expected van't Hoff factor
Q116: What is the expected van't Hoff factor
Q117: Choose the aqueous solution that has the
Q117: To make a 2.00 mol kg-1 solution,one
Q119: A solution of methanol and water has
Q120: Choose the aqueous solution below with the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents