Consider the molecule below. Determine the molecular geometry at each of the three labelled atoms. 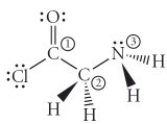
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal
Correct Answer:
Verified
Q7: Place the following in order of decreasing
Q13: Determine the electron geometry (eg)and molecular geometry
Q45: Determine the electron geometry (eg) and molecular
Q46: Consider the molecule below. Determine the molecular
Q47: Determine the molecular geometry (mg) of the
Q48: Determine the electron geometry (eg) and molecular
Q50: Determine the electron geometry (eg) and molecular
Q52: Determine the molecular geometry (mg) of the
Q53: Determine the molecular geometry (mg) of the
Q53: Determine the molecular geometry (mg) of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents