According to the following reaction, how much energy is evolved during the reaction of 2.50 L B2H6 and 5.65 L Cl2 (both gases are initially at STP) ? The molar mass of B2H6 is 27.67 g 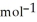 . B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g)
. B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g) 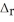 H° = -1396 kJ
H° = -1396 kJ
A) 57.8 kJ
B) 156 kJ
C) 215 kJ
D) 352 kJ
E) 508 kJ
Correct Answer:
Verified
Q73: Calculate the heat transfer, in kJ, when
Q74: Calculate the heat transfer (J) when 3.15
Q75: What volume of benzene (C6H6, d =
Q76: Calculate the final temperature of 68.4 g
Q77: An unknown metal alloy, mass = 26.3
Q79: Identify what a coffee cup calorimeter measures.
A)
Q80: According to the following thermochemical equation, what
Q81: Calculate the enthalpy of solution for the
Q82: An unknown metal alloy, specific heat capacity
Q83: Calculate the final temperature of a 38.1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents