According to the following thermochemical equation, what mass of HF (in g) must react to produce 345 kJ of energy? Assume excess SiO2. SiO2(s) + 4HF(g) → SiF4(g) + 2H2O(l) 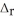 H° = -184 kJ
H° = -184 kJ
A) 42.7 g
B) 37.5 g
C) 150. g
D) 107 g
E) 173 g
Correct Answer:
Verified
Q75: What volume of benzene (C6H6, d =
Q76: Calculate the final temperature of 68.4 g
Q77: An unknown metal alloy, mass = 26.3
Q78: According to the following reaction, how much
Q79: Identify what a coffee cup calorimeter measures.
A)
Q81: Calculate the enthalpy of solution for the
Q82: An unknown metal alloy, specific heat capacity
Q83: Calculate the final temperature of a 38.1
Q84: A piece of gold with a mass
Q85: Use the standard reaction enthalpies given below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents