Using the following equation for the combustion of octane, calculate the amount of grams of carbon dioxide formed from 100.0 g of octane. The molar mass of octane is 114.33 g mol-1. The molar mass of carbon dioxide is 44.0095 g mol-1. 2C8H18 + 25O2 → 16CO2 + 18H2O 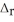 H° = -11018 kJ
H° = -11018 kJ
A) 800.1 g
B) 307.9 g
C) 260.1 g
D) 792.3 g
Correct Answer:
Verified
Q66: Calculate the final temperature of 82.1 g
Q67: Astatine is an extremely rare element with
Q68: Two aqueous solutions are both at room
Q69: Calculate the initial temperature of 448 g
Q70: Calculate the final temperature of 6.84 g
Q72: Calculate the initial temperature of 648 g
Q73: Calculate the heat transfer, in kJ, when
Q74: Calculate the heat transfer (J) when 3.15
Q75: What volume of benzene (C6H6, d =
Q76: Calculate the final temperature of 68.4 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents