Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter. The reaction causes the temperature of the resulting solution to fall below room temperature. Which of the following statements is TRUE?
A) The products have a lower potential energy than the reactants.
B) This type of experiment will provide data to calculate 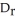 U.
U.
C) The reaction is exothermic.
D) Energy is leaving the system during the reaction.
E) None of the above statements is true.
Correct Answer:
Verified
Q63: An unknown metal alloy, mass = 36.1
Q64: According to the following thermochemical equation, what
Q65: Calculate the initial temperature of 21.8 g
Q66: Calculate the final temperature of 82.1 g
Q67: Astatine is an extremely rare element with
Q69: Calculate the initial temperature of 448 g
Q70: Calculate the final temperature of 6.84 g
Q71: Using the following equation for the combustion
Q72: Calculate the initial temperature of 648 g
Q73: Calculate the heat transfer, in kJ, when
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents