According to the following thermochemical equation, what mass of H2O (in g) must form to produce 975 kJ of energy? SiO2(s) + 4HF(g) → SiF4(g) + 2H2O(l) 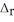 H° = -184 kJ
H° = -184 kJ
A) 68.0 g
B) 102 g
C) 54.1 g
D) 191 g
E) 95.5 g
Correct Answer:
Verified
Q59: A 12.8 g sample of ethanol (C2H5OH)
Q60: A bomb calorimeter with a heat capacity
Q61: Which of the following statements is TRUE?
A)
Q62: Calculate the heat transfer, in J, when
Q63: An unknown metal alloy, mass = 36.1
Q65: Calculate the initial temperature of 21.8 g
Q66: Calculate the final temperature of 82.1 g
Q67: Astatine is an extremely rare element with
Q68: Two aqueous solutions are both at room
Q69: Calculate the initial temperature of 448 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents