10.618 g of an unknown salt is dissolved in a coffee cup calorimeter that contains 50.0 mL of water. The measured temperature decreased from 23.8 °C to 22.7 °C. Identify the unknown salt. The specific heat of water is 4.184 J g-1 °C-1, and assume the density of the solution is 1.00 g mL-1.
A) NaF, 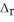 H = 0.91 kJ mol-1
H = 0.91 kJ mol-1
B) NaBr, 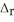 H = -0.60 kJ mol-1
H = -0.60 kJ mol-1
C) NaNO3, 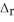 H = 20.50 kJ mol-1
H = 20.50 kJ mol-1
D) LiNO3, 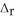 H = -2.51 kJ mol-1
H = -2.51 kJ mol-1
E) LiBr∙2H2O, 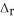 H = -23.26 kJ mol-1
H = -23.26 kJ mol-1
Correct Answer:
Verified
Q88: Two solutions, initially at 24.69 °C, are
Q89: A piece of iron (mass = 25.0
Q90: Calculate the mass of sodium cyanate (NaOCN,
Q91: Calculate the mass of NH4ClO4, molar mass
Q92: An unknown metal alloy, specific heat capacity
Q94: Use the standard reaction enthalpies given below
Q95: Use the standard reaction enthalpies given below
Q96: Calculate the final temperature of a 63.7
Q97: A 100.0 mL sample of 0.300 mol
Q98: A student is preparing to perform a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents