Use the standard reaction enthalpies given below to determine 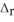 H° for the following reaction: 2NO(g) + O2(g) → 2NO2(g)
H° for the following reaction: 2NO(g) + O2(g) → 2NO2(g) 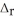 H° = ?
H° = ?
Given:
N2(g) + O2(g) → 2NO(g) 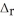 H° = +183 kJ
H° = +183 kJ
1/2 N2(g) + O2(g) → NO2(g) 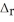 H° = +33 kJ
H° = +33 kJ
A) -150. kJ mol-1
B) -117 kJ mol-1
C) -333 kJ mol-1
D) +115 kJ mol-1
E) +238 kJ mol-1
Correct Answer:
Verified
Q89: A piece of iron (mass = 25.0
Q90: Calculate the mass of sodium cyanate (NaOCN,
Q91: Calculate the mass of NH4ClO4, molar mass
Q92: An unknown metal alloy, specific heat capacity
Q93: 10.618 g of an unknown salt is
Q95: Use the standard reaction enthalpies given below
Q96: Calculate the final temperature of a 63.7
Q97: A 100.0 mL sample of 0.300 mol
Q98: A student is preparing to perform a
Q99: Calculate the enthalpy of solution for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents