Multiple Choice
Use the 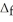 H° and
H° and 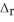 H° information provided to calculate
H° information provided to calculate 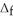 H° for SO3(g) :
H° for SO3(g) : 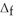 H° (kJ
H° (kJ 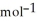 ) 2SO2(g) + O2(g) → 2SO3(g)
) 2SO2(g) + O2(g) → 2SO3(g) 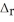 H° = -198 kJ SO2(g) -297
H° = -198 kJ SO2(g) -297
A) -792 kJ mol-1
B) -248 kJ mol-1
C) -495 kJ mol-1
D) -578 kJ mol-1
E) -396 kJ mol-1
Correct Answer:
Verified
Related Questions
Q103: Identify a substance that is in a
Q104: Use the information provided to determine
Q105: Calculate the kinetic energy of a 150
Q106: Identify the greatest source of energy worldwide.
A)
Q107: Choose the reaction that illustrates ΔH° for
Q109: Use the information provided to determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents