Use the information provided to determine 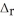 H° for the following reaction:
H° for the following reaction: 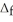 H°(kJ
H°(kJ 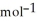 ) CH4(g) + 4 Cl2(g) → CCl4(g) + 4 HCl(g)
) CH4(g) + 4 Cl2(g) → CCl4(g) + 4 HCl(g) 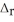 H° = ? CH4(g) -75
H° = ? CH4(g) -75
CCl4(g) -96
HCl(g) -92
A) -389 kJ mol-1
B) -113 kJ mol-1
C) +113 kJ mol-1
D) -71 kJ mol-1
E) +79 kJ mol-1
Correct Answer:
Verified
Q99: Calculate the enthalpy of solution for the
Q100: Two solutions, initially at 24.60 °C, are
Q101: Choose the reaction that illustrates ΔH° for
Q102: Use the standard reaction enthalpies given below
Q103: Identify a substance that is in a
Q105: Calculate the kinetic energy of a 150
Q106: Identify the greatest source of energy worldwide.
A)
Q107: Choose the reaction that illustrates ΔH° for
Q108: Use the Q109: Use the information provided to determine ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents