Use the information provided to determine 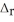 H° for the following reaction:
H° for the following reaction: 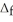 H° (kJ
H° (kJ 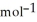 ) 3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g)
) 3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g) 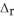 H° = ? Fe2O3(s) -824
H° = ? Fe2O3(s) -824
Fe3O4(s) -1118
CO(g) -111
CO2(g) -394
A) +277 kJ mol-1
B) -577 kJ mol-1
C) -47 kJ mol-1
D) +144 kJ mol-1
E) -111 kJ mol-1
Correct Answer:
Verified
Q109: Use the information provided to determine
Q110: Use the Q111: Choose the thermochemical equation that illustrates ΔH° Q112: For a process at constant pressure, 62,000 Q113: Identify the major greenhouse gas. Q115: Which of the following is not a![]()
A) CO2
B) O2
C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents