Multiple Choice
Which of the following processes is exothermic?
A) the ionization of a lithium atom
B) the breaking of a Cl-Cl bond
C) the sublimation of dry ice (CO2(s) )
D) the reaction associated with 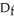 H° for an ionic compound
H° for an ionic compound
Correct Answer:
Verified
Related Questions
Q113: Identify the major greenhouse gas.
A) CO2
B) O2
C)
Q114: Use the information provided to determine
Q115: Which of the following is not a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents